Balmer's Formula
 What was the formula that Balmer found?
What was the formula that Balmer found?
 Balmer examined the four visible lines in the spectrum of the hydrogen atom; their wavelengths are
410 nm, 434 nm, 486 nm, and 656 nm. He played around with these numbers and eventually figured out
that all four wavelengths (symbolized by the Greek letter lambda) fit into the equation
Balmer examined the four visible lines in the spectrum of the hydrogen atom; their wavelengths are
410 nm, 434 nm, 486 nm, and 656 nm. He played around with these numbers and eventually figured out
that all four wavelengths (symbolized by the Greek letter lambda) fit into the equation
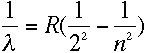
|
|
R is the Rydberg constant, whose value is
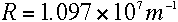
| The number n is just an integer; the above formula gives the longest wavelength, 656 nm, when n=3, and gives each of the shorter wavelengths as n increases up to 6. |

 656 nm is the red line in that picture, right?
656 nm is the red line in that picture, right?
 Exactly; the shorter wavelengths correspond to the blue and violet lines you can see. (The 410 nm
line is very faint, but it's there if you look closely.) This set of lines is called the Balmer
series. Later, other researchers found that the series could be extended into ultraviolet
wavelengths; the same formula still worked, with larger values of n.
Exactly; the shorter wavelengths correspond to the blue and violet lines you can see. (The 410 nm
line is very faint, but it's there if you look closely.) This set of lines is called the Balmer
series. Later, other researchers found that the series could be extended into ultraviolet
wavelengths; the same formula still worked, with larger values of n.
 Hmm...from Balmer's equation, it looks like when n gets bigger, the lines should start
getting really close together.
Hmm...from Balmer's equation, it looks like when n gets bigger, the lines should start
getting really close together.
 That's exactly right; as n gets larger, 1 over n squared gets smaller, so there's less
and less difference between the consecutive lines. You can see that the series has a limit--
that is, as n gets larger and larger, the wavelength gets closer and closer to one particular
value. If n is infinity, then 1 over n squared is 0, and if you work out the numbers,
you'll find that the wavelength is about 365 nm. That's just what experimentalists saw; around
365 nm, the lines became too close together to distinguish.
That's exactly right; as n gets larger, 1 over n squared gets smaller, so there's less
and less difference between the consecutive lines. You can see that the series has a limit--
that is, as n gets larger and larger, the wavelength gets closer and closer to one particular
value. If n is infinity, then 1 over n squared is 0, and if you work out the numbers,
you'll find that the wavelength is about 365 nm. That's just what experimentalists saw; around
365 nm, the lines became too close together to distinguish.
 So do all of the emission lines from a hydrogen atom fit somewhere into the Balmer series?
So do all of the emission lines from a hydrogen atom fit somewhere into the Balmer series?
 No, they don't. As scientists looked further into the non-visible parts of the spectrum, they
found other series--which obeyed formulas hauntingly similar to Balmer's. For example, the
Lyman series, which is entirely in the ultraviolet, fits the equation
No, they don't. As scientists looked further into the non-visible parts of the spectrum, they
found other series--which obeyed formulas hauntingly similar to Balmer's. For example, the
Lyman series, which is entirely in the ultraviolet, fits the equation
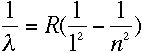
|
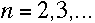
|
| And the lines of the Paschen series, in the infrared, fit |
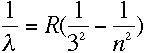
|
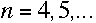
|
 Those integers must have something to do with Bohr's energy levels.
Those integers must have something to do with Bohr's energy levels.
 Yes, they do; I'll show you how Bohr was able to deduce the electron's angular momentum from this formula...
Yes, they do; I'll show you how Bohr was able to deduce the electron's angular momentum from this formula...





