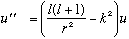
Physics 3220, Fall '97. Steve Pollock.
Here is the previous lecture
Here is the Next lecture
Back to the list of lectures
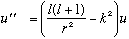
is
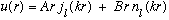
where j_l is the spherical Bessel fn, and n_l is the spherical Neumann fn.
The functions can be found by the following general formulae:
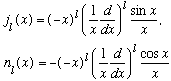
or more specifically,
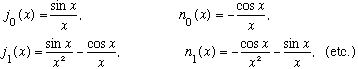
Note that if you set l=0, you get u=Asin(kr)-Bcos(kr), just as we had found.
Spherical Bessels are finite at r=0, but Neumann's blow up like 1/r^(l+1)
So the solution, r j_l(kr) goes to 0 nicely as r -> 0, but r n_l(kr) is finite (or infinite) as r->0, neither of which is acceptable. Thus, we must have B=0.
Our solution is then
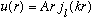
u(a)=0 still must be true. So plugging this is we find A a j_l(ka) =0.
Thus, k is constrained. Indeed, ka must be a zero of the lth spherical Bessel.
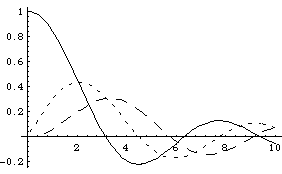
Here are plots of j0 (solid),
j1 (short dash), and
j2 (long dash)
These fns are kind of "sin"-ey, but not exactly. They do have an infinite number of zeros, but the function damps away, and the zeros are not evenly spaced out (except l=0.)
If you call the nth zero of the l'th Bessel function
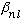 ,
we have
,
we have
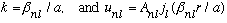 .
.
Finally, the total wave function is
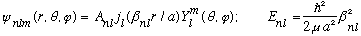
(The A_nl can be found by normalizing. )
Note that (Boas proves this) if n' is different from n, then
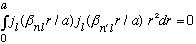
Which says the wave functions are orthogonal if n and n' differ, even if l and m are the same. (If l and/or m differ, the wave functions will be orthogonal due to the angular integration over the Ylm's. )
The lowest zero of any of the Bessel fns is
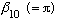 .
(Because this is l=0, we call it an "S" state, and this is the
first out of all the Bessel's zeros.) The next lowest zero of any
Bessel fn is
.
(Because this is l=0, we call it an "S" state, and this is the
first out of all the Bessel's zeros.) The next lowest zero of any
Bessel fn is
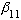 (Because this is l=1, we call it a "P" state. This is the first zero of
the l=1 Bessel.) The next zero of any Bessel fns is
(Because this is l=1, we call it a "P" state. This is the first zero of
the l=1 Bessel.) The next zero of any Bessel fns is
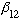 (
l=2, "D" state, first crossing.) Before you think you can predict the
pattern, beware. The next zero of all the Bessel functions is now the
second zero of the l=0, or "S" state,
(
l=2, "D" state, first crossing.) Before you think you can predict the
pattern, beware. The next zero of all the Bessel functions is now the
second zero of the l=0, or "S" state,
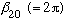 .
.
These results tell you what happens if you start sticking electrons into an infinite, spherical box (or, roughly, protons and neutrons into a nucleus!)
You will first fill up the 1s state. This is non-degenerate. (Spin => 2 protons)
Then you fill up the 1p state. (3 fold degenerate, 6 protons. Total is now 8)
Then the 1d state. (5 fold, 10 protons, total is now 18). Next, comes the 2s state (non-degenerate, 2 protons more => 20) These are "Magic nuclear #'s"!
Next example: A finite square well in 3-D
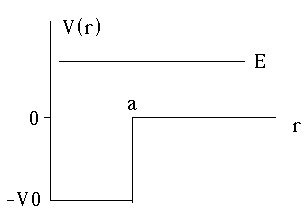
So, V(r) = -V0 (r<a),
0 (r>a)
As usual, in 3-D, the effective potential goes to infinity at r=0, so as to force u(r=0) to 0.
We will first consider E>0. This means free particles, or continuum solutions.
We know from 1-D that E>0 means E is not quantized. You can pick it!
The physics here is of two attractive particles which scatter from one another. (Or, a single particle hitting a fixed attractive center, and scattering off it)
The radial equation, as usual, looks like
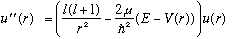
When r<a, we define
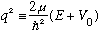
Then the radial equation looks just like the one we solved in the previous example (except for the switch of symbol k for symbol q)!
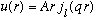 if r<a.
if r<a.
(The n_l solution is, as before, no good when r -> 0)
When r>a, we define
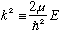 ,
and again the radial equation looks just like the one we solved in the
previous example, and
,
and again the radial equation looks just like the one we solved in the
previous example, and
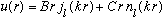 when r>a.
when r>a.
Note that we have no reason to throw away the n_l term this time, because this solution is only valid for r>a, so there's absolutely nothing wrong with the n_l function!
Now, let's switch back to R(r)= u(r)/r, to get ride of the irritating "r" factors (remember, R(r) is the true radial wavefunction anyway, we just defined u(r) so that our effective radial equation would look like the old 1-D one.)
Of course, R(r) must be continuous everywhere (including r=a), and the same goes for R'(r). (Just like in 1-D, otherwise the S.E. doesn't make sense)
We will name the solution when r<a, "R<", and the solution for r>a, "R>".
Next time, we will show what continuity of R and R' says.
Here is the Next lecture
| 3220 main page | Prof. Pollock's page. | Physics Dep't | Send comments |